නිකොටින්
Appearance
උ
| |
|---|---|

| |
| Systematic (IUPAC) name | |
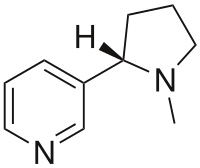
| (S)-3-[1-Methylpyrrolidin-2-yl]pyridine | |
| Clinical data | |
| Trade names | Nicorette, Nicotrol |
| AHFS/Drugs.com | Monograph |
| Pregnancy cat. | D (AU) D (US) |
| Legal status | Pharmacy Only (S2) (AU) GSL (UK) OTC (US) |
| Dependence liability | High |
| Routes | Inhalation; Insufflation; Oral – Buccal, Sublingual, and Ingestion; Transdermal; Rectal, |
| Pharmacokinetic data | |
| Bioavailability | 20 to 45% (oral), 53% (intranasal), 68% (transdermal) |
| Protein binding | <5% |
| Metabolism | Hepatic |
| Half-life | 1-2 hours; 20 hours active metabolite (cotinine) |
| Excretion | Urine (10-20% (gum), pH-dependent; 30% (inhaled); 10-30% (intranasal)) |
| Identifiers | |
| CAS number | 54-11-5 |
| ATC code | N07BA01 සැකිල්ල:ATCvet |
| PubChem | CID 89594 |
| IUPHAR ligand | 2585 |
| DrugBank | DB00184 |
| ChemSpider | 80863 |
| UNII | 6M3C89ZY6R |
| KEGG | D03365 |
| ChEBI | CHEBI:18723 |
| ChEMBL | CHEMBL3 |
| Chemical data | |
| Formula | C10H14N2 |
| Mol. mass | 162.12 g/mol |
| |
| |
| Physical data | |
| Density | 1.01 g/cm³ |
| Melt. point | -79 °C (-110 °F) |
| Boiling point | 247 °C (477 °F) |
| | |
Nicotine is a potent parasympathomimetic alkaloid found in the nightshade family of plants (Solanaceae) and a stimulant drug. It is a nicotinic acetylcholine receptor agonist. It is made in the roots and accumulates in the leaves of the plants. It constitutes approximately 0.6–3.0% of the dry weight of tobacco[1] and is present in the range of 2–7 µg/kg of various edible plants.[2] It functions as an antiherbivore chemical; consequently, nicotine was widely used as an insecticide in the past[3][4]
- ^ "Smoking and Tobacco Control Monograph No. 9" (PDF). සම්ප්රවේශය 2012-12-19.
- ^ "Determination of the Nicotine Content of Various Edible Nightshades (Solanaceae) and Their Products and Estimation of the Associated Dietary Nicotine Intake". සම්ප්රවේශය 2008-10-05.
- ^ Rodgman, Alan; Perfetti, Thomas A. (2009). The chemical components of tobacco and tobacco smoke. Boca Raton, FL: CRC Press. ISBN 1-4200-7883-6. LCCN 2008018913.[page needed]
- ^ Ujváry, István (1999). "Nicotine and Other Insecticidal Alkaloids". In Yamamoto, Izuru; Casida, John (eds.). Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo: Springer-Verlag. pp. 29–69.
