බන්ඩි පෝර
Appearance
(Potassium chloride වෙතින් යළි-යොමු කරන ලදි)
සැකිල්ල:Chembox Excretion

| |
| |
| Names | |
|---|---|
| වෙනත් නාම
Sylvite
Muriate of potash | |
| Identifiers | |
| CAS number | {{{value}}} |
3D model (JSmol)
|
|
| ChEBI | CHEBI:{{{value}}} |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | {{{value}}} |
| PubChem | {{{value}}} |
| RTECS number | {{{value}}} |
| UNII | |
| InChI | |
| SMILES | |
| Properties | |
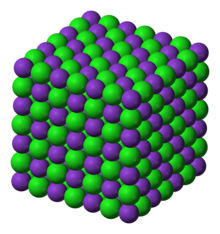
| Molecular formula | KCl |
| අණුක ස්කන්ධය | 74.555 g·mol−1 |
| Appearance | white crystalline solid |
| Odor | odorless |
| Density | 1.984 g/cm3 |
| Melting point |
770 °C, 1043 K, 1418 °F |
| Boiling point |
1420 °C, 1693 K, 2588 °F |
| Solubility in water | 27.77 g/100mL (0 °C) 33.97 g/100mL (20 °C) 54.02 g/100mL (100 °C) |
| Solubility | Soluble in glycerol, alkalies Slightly soluble in alcohol Insoluble in ether[1] |
| Solubility in ethanol | 0.288 g/L (25 °C)[2] |
| Acidity (pKa) | ~7 |
| −39.0·10−6 cm3/mol | |
| Solubility product, Ksp | 1.4902 (589 nm) |
| Structure | |
| Crystal structure | face centered cubic |
| Space group | Fm3m, No. 225 |
| Coordination geometry |
Octahedral (K+) Octahedral (Cl−) |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−436 kJ·mol−1[4] |
Std molar
entropy (S⦵298) |
83 J·mol−1·K−1[4] |
| Pharmacology | |
| Oral, IV, IM | |
| Hazards | |
| Flash point | {{{value}}} |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2600 mg/kg (oral, rat)[6] |
| Safety data sheet (SDS) | ICSC 1450 |
| Related compounds | |
| Other anions | Potassium fluoride Potassium bromide Potassium iodide |
| Other cations | Lithium chloride Sodium chloride Rubidium chloride Caesium chloride Ammonium chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
ආශ්රිත ලිපි
[සංස්කරණය]- ^ "Potassium chloride (PIM 430)". International Programme on Chemical Safety. 3.3.1 Properties of the substance. සම්ප්රවේශය 2011-01-17.
- ^ "periodic-table-of-elements.org". 29 October 2020 දින මුල් පිටපත (website shows values in g/100ml) වෙතින් සංරක්ෂණය කරන ලදී. සම්ප්රවේශය 4 October 2019.
- ^ Sirdeshmukh DB, Sirdeshmukh L, Subhadra KG (2001). Alkali Halides: A Handbook of Physical Properties. Berlin: Springer. ISBN 978-3-540-42180-1.
- ^ a b Zumdahl SS (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ "Compound Summary for Potassium Chloride". PubChem. U.S. National Library of Medicine. CID 4873. සම්ප්රවේශය 17 October 2015.
- ^ Chambers M. "7447-40-7 - WCUXLLCKKVVCTQ-UHFFFAOYSA-M - Potassium chloride [USP:JAN]". ChemIDplus. U.S. National Library of Medicine. 15 July 2015 දින මුල් පිටපත වෙතින් සංරක්ෂණය කරන ලදී. සම්ප්රවේශය 22 December 2017.
