ඇසිටිලින්
Appearance
(ඇසිටිලින් වායුව වෙතින් යළි-යොමු කරන ලදි)

| |

| |
| Names | |
|---|---|
| IUPAC name
Acetylene[1]
| |
| Systematic IUPAC name
Ethyne | |
| Identifiers | |
| CAS number | {{{value}}} |
3D model (JSmol)
|
|
| ChEBI | CHEBI:{{{value}}} |
| ChemSpider | |
| DrugBank | |
| KEGG | {{{value}}} |
| PubChem | {{{value}}} |
| RTECS number | {{{value}}} |
| UNII | |
| UN number | 1001 (dissolved) 3138 (in mixture with ethylene and propylene) |
| InChI | |
| SMILES | |
| Properties | |
| Molecular formula | C2H2 |
| Molar mass | 26.04 g mol−1 |
| Density | 1.097 kg/m3 |
| Melting point |
−80.8 °C (189 K, subl) |
| Boiling point |
−84 °C |
| Acidity (pKa) | 25 |
| Structure | |
| Molecular shape | Linear |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
+226.88 kJ/mol |
| Hazards | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Reactions
[සංස්කරණය]මූලික ලිපිය: Alkyne
Reppe chemistry
[සංස්කරණය]Walter Reppe discovered that in the presence of metal catalysts, acetylene can react to give a wide range of industrially significant chemicals.
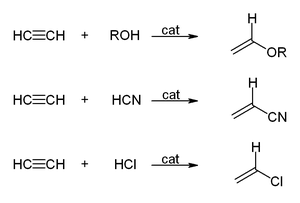
- With alcohols, hydrogen cyanide, hydrogen chloride, or carboxylic acids to give vinyl compounds:
- With aldehydes to give ethynyl diols.
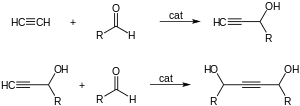
- 1,4-Butynediol is produced industrially in this way from formaldehyde and acetylene.
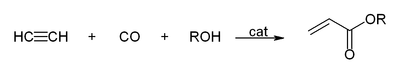
- With carbon monoxide to give acrylic acid, or acrylic esters, which can be used to produce acrylic glass.
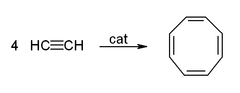
- Cyclicization to give benzene and cyclooctatetraene:
References
[සංස්කරණය]- ^ Acyclic Hydrocarbons. Rule A-3. Unsaturated Compounds and Univalent Radicals, IUPAC Nomenclature of Organic Chemistry